The safety and efficacy of medical devices and pharmaceutical products are paramount concerns within the healthcare industry. Even with rigorous pre-market testing and clinical trials, the dynamic nature of real-world usage necessitates continuous monitoring once these products are available to the public. This ongoing process is known as post-market surveillance (PMS), and it serves as a critical safety net, ensuring that medical interventions remain beneficial for the diverse populations that utilize them. The initial assessments conducted before a product’s release, while providing valuable insights, often involve limited sample sizes and controlled environments, which may not fully capture the spectrum of potential issues that can arise in everyday clinical practice. Therefore, PMS plays an indispensable role in gathering real-world data to validate the continued safety and utility of medical products.
Traditionally, PMS has relied heavily on manual methods for collecting, analyzing, and interpreting data related to product performance and adverse events. This often involves the painstaking review of case reports, complaints, and other forms of feedback submitted by healthcare professionals, patients, and other stakeholders. However, the sheer volume of data generated in today’s healthcare landscape presents a significant challenge to these traditional approaches. With an increasing number of medical devices and drugs on the market, coupled with more accessible reporting mechanisms like electronic health records and online platforms, the amount of information requiring analysis has grown exponentially. This surge in data can overwhelm manual systems, potentially leading to delays in identifying critical safety signals and hindering timely interventions. The task of manually sifting through countless reports to pinpoint potential issues can be likened to searching for a specific piece of information within an ever-expanding library, a process that is both time-consuming and prone to human error. Consequently, the need for more advanced and efficient methods of post-market surveillance has become increasingly apparent.

The emergence of artificial intelligence (AI) offers a transformative solution to the challenges faced by traditional PMS methods. AI technologies possess the capability to process and analyze vast datasets at remarkable speeds, identifying subtle patterns and correlations that might escape human detection. This ability to rapidly analyze large volumes of information provides a significant advantage in the context of PMS, where the timely identification of safety signals is crucial for patient well-being. Imagine a system that can continuously scan and evaluate millions of data points, flagging potential concerns with an efficiency that far surpasses human capacity. This is the promise of AI in revolutionizing post-market surveillance.
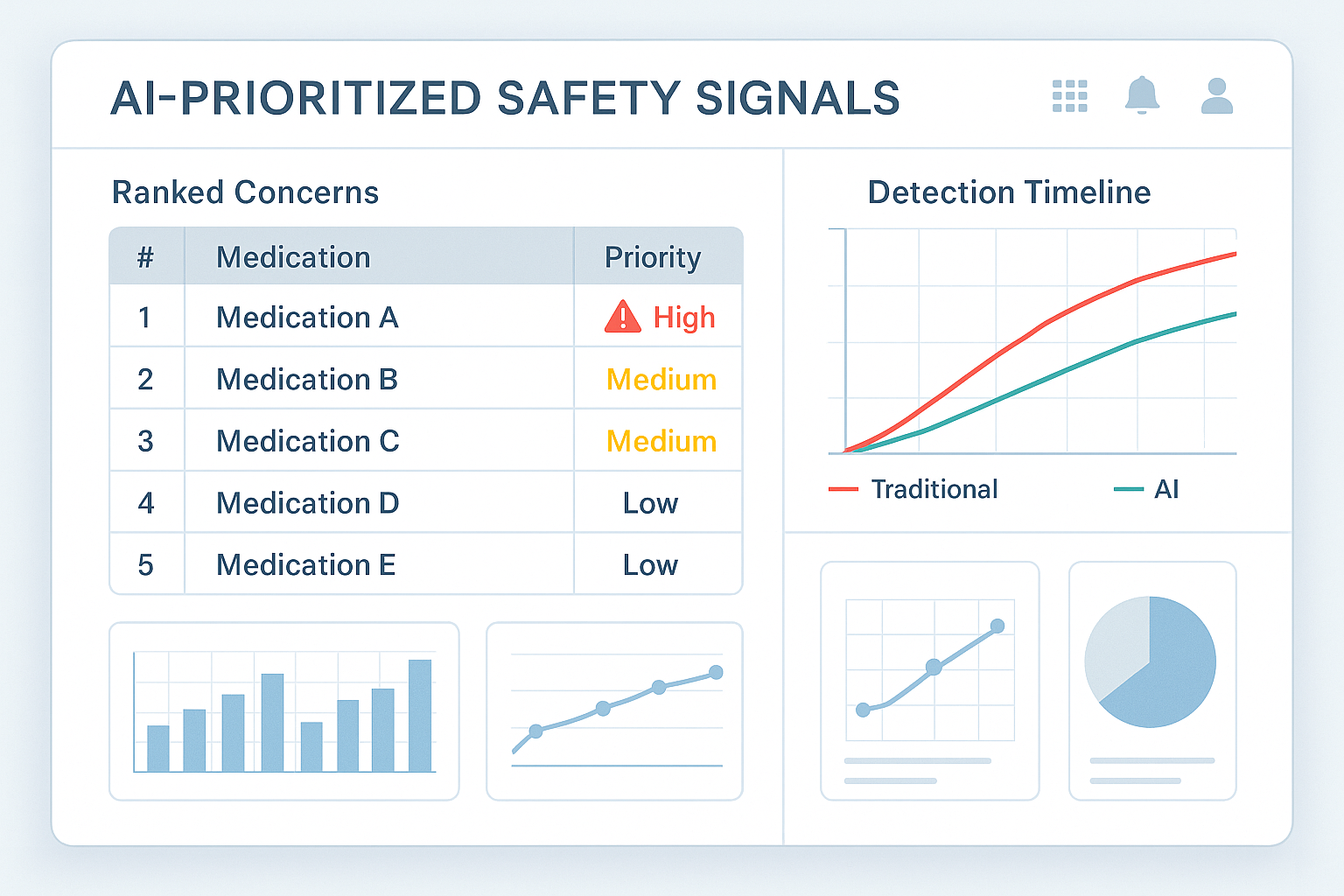
Specifically, the application of neural networks within AI systems has demonstrated remarkable potential in enhancing signal prioritization [User Query]. These complex algorithms, modeled after the structure and function of the human brain, can be trained on extensive datasets to learn intricate patterns and relationships within the data. The user query highlights a compelling example of this technology in action: neural networks processing an astounding 1.2 million global case reports on a weekly basis to autonomously rank signals according to their clinical urgency [User Query]. This sophisticated system was able to escalate concerns regarding a novel insomnia medication’s link to complex sleep behaviors a remarkable 83 days faster than traditional manual processes [User Query]. This substantial reduction in detection time underscores the profound impact that AI-driven systems can have on expediting safety interventions and ultimately improving patient outcomes. The ability to identify potential risks associated with medications or medical devices in a significantly shorter timeframe can lead to quicker investigations, more informed regulatory decisions, and ultimately, enhanced patient safety.
To understand how AI achieves this level of efficiency, it’s essential to delve into the underlying mechanisms of these advanced architectures. At the heart of many AI-driven PMS systems are neural networks, intricate computational models inspired by the neural structure of the human brain. These networks consist of interconnected nodes, or artificial neurons, organized in layers. Through a process called training, these networks learn to recognize patterns in data by being exposed to vast amounts of historical information. In the context of PMS, this training data often includes a multitude of case reports detailing adverse events, product malfunctions, and other relevant information. By analyzing these historical examples, the neural network gradually adjusts the connections between its nodes, allowing it to identify specific features and combinations of factors that are indicative of potential safety issues.
Consider the analogy of teaching a child to distinguish between different types of fruits. You might show the child numerous examples of apples, bananas, and oranges, highlighting their distinct characteristics such as shape, color, and texture. Over time, the child learns to recognize these fruits based on the patterns of features they have observed. Similarly, a neural network is trained on a large dataset of PMS-related information, learning to identify patterns in symptoms, medications, patient demographics, and outcomes that may signal a safety concern. This learning process enables the AI system to analyze new, incoming data and identify potential issues with a level of accuracy and speed that would be difficult for humans to achieve manually.
Furthermore, many AI systems employed in PMS leverage the power of natural language processing (NLP). Case reports and other forms of feedback often contain unstructured text data, where information is conveyed through narrative descriptions rather than standardized fields. NLP techniques enable AI to understand and extract key information from this free-form text, identifying crucial details such as reported symptoms, medications used, and patient outcomes. This capability is particularly valuable in PMS, as a significant portion of the data resides in unstructured formats. By processing and interpreting the nuances of human language, AI can effectively transform this textual information into a structured format that can be further analyzed to detect potential safety signals. This allows the AI system to not only process numerical data but also to comprehend the rich information contained within narrative reports, leading to a more comprehensive and insightful analysis of post-market safety.

Beyond simply identifying potential problems, a crucial aspect of effective post-market surveillance involves understanding the overall benefit-risk profile of a medical product. This requires weighing the therapeutic benefits a product offers against any potential safety risks it may pose. Integrated benefit-risk analytics platforms play a vital role in this process. These sophisticated platforms often utilize multi-criteria decision analysis (MCDA), a structured approach that considers various factors, including the efficacy of the treatment and any emerging safety signals. By simultaneously evaluating these different criteria, MCDA provides a more holistic understanding of a product’s overall impact.
The user query provides a compelling illustration of this integrated approach in the context of psoriasis biologics in 2025 [User Query]. By incorporating patient-reported quality-of-life metrics into the benefit-risk analysis, the assessment went beyond traditional clinical outcomes to consider the patient’s overall well-being [User Query]. This more patient-centric approach led to the development of personalized treatment algorithms, enabling healthcare professionals to make more informed decisions about the most appropriate treatment for each individual patient [User Query]. By considering not only the medical effectiveness of a treatment but also its impact on a patient’s daily life and overall quality of life, these integrated platforms facilitate a more nuanced and tailored approach to healthcare decision-making. This example underscores the growing recognition of the importance of incorporating the patient’s perspective into the evaluation of medical products, moving towards a more holistic and personalized approach to treatment.
The application of AI in PMS is not merely a theoretical concept; it is actively being implemented in various practical scenarios, yielding tangible benefits in enhancing medical product safety. Several examples from the provided information highlight the diverse ways in which AI is currently being utilized.
AI algorithms are proving invaluable in the detection of safety signals from a wide array of data sources. This includes the analysis of real-world data collected from implantable cardiac devices, where AI can identify patterns indicative of potential malfunctions or adverse events. Similarly, AI systems are being used to categorize and classify adverse events associated with surgical instruments, enabling more efficient identification of trends and potential safety concerns. Furthermore, AI’s capability to analyze unstructured data, such as social media posts and electronic health records, expands the scope of signal detection beyond traditional reporting systems, potentially uncovering safety issues that might otherwise go unnoticed. This ability to monitor diverse channels for safety-related information provides a more comprehensive and timely understanding of a medical product’s performance in the real world.
In the realm of risk assessment and prioritization, AI tools offer significant advantages. By analyzing the strength of evidence and assessing the potential clinical urgency of reported events, AI algorithms can rank and prioritize safety signals, allowing pharmacovigilance teams to focus their resources on the most critical issues. For instance, the World Health Organization’s VigiBase utilizes VigiRank, a machine learning-based algorithm, to rank adverse drug reaction signals based on various criteria, facilitating early identification of the most pressing safety concerns. This intelligent prioritization ensures that the most serious potential risks are addressed promptly, improving the overall efficiency and effectiveness of PMS activities.
AI is also playing a crucial role in improving regulatory compliance within the PMS domain. By automating the compilation and formatting of essential regulatory documents, such as periodic safety update reports, AI can save valuable time and reduce the risk of errors, ensuring timely and accurate submissions to regulatory authorities. This automation not only streamlines the regulatory process but also minimizes the potential for non-compliance due to administrative oversights.
The analysis of case reports is another area where AI is making significant contributions. Neural networks and other AI techniques are being employed to extract valuable insights and identify patterns within these reports that may indicate a safety concern. For example, AI can be used to automatically code adverse events and assess their severity based on the information contained in the free text of patient reports. This automated analysis enhances the efficiency of pharmacovigilance activities and can help uncover potential drug-event relationships that might be missed through manual review.
While not directly related to the post-market surveillance of a specific medical device or pharmaceutical, the advancements in using AI, including neural networks, in sleep medicine offer a compelling example of AI’s potential to monitor complex physiological data. AI algorithms are being used to analyze sleep patterns, detect sleep disorders like REM sleep behavior disorder, and monitor the effectiveness of treatments. Given the user query’s mention of an insomnia medication and its association with complex sleep behaviors, these advancements in sleep medicine highlight the potential of AI to monitor for specific adverse events related to certain medications or devices. The ability of AI to analyze intricate physiological data and identify anomalies could be directly applicable to monitoring and detecting complex sleep behaviors reported as potential side effects of insomnia treatments.
| Application Area | Description | Relevant Snippet IDs |
| Signal Detection from Diverse Data Sources | AI algorithms analyze various data types (e.g., device data, social media, EHRs) to identify potential safety concerns. | 9 |
| Risk Assessment and Prioritization | AI tools rank safety signals based on evidence strength and clinical urgency, aiding in focused resource allocation. | 8 |
| Improving Regulatory Compliance | AI assists in automating the compilation and formatting of regulatory reports, ensuring timely and accurate submissions. | 9 |
| Analyzing Case Reports | AI techniques like neural networks and NLP are used to extract insights and identify patterns in adverse event reports. | 12 |
| Monitoring Complex Behaviors | AI analyzes physiological data (e.g., sleep patterns) to detect specific adverse events or monitor treatment effectiveness. | 26 |
While the integration of AI into PMS offers numerous advantages, it is important to acknowledge the challenges and ethical considerations that accompany this technological advancement. Data privacy is a paramount concern, as the analysis of large datasets containing patient information necessitates robust safeguards to protect sensitive data. Algorithm bias is another critical consideration; if the data used to train AI algorithms is not representative of the entire patient population, it could lead to skewed or unfair outcomes. Ensuring transparency and interpretability of AI outputs is also crucial, as healthcare professionals need to understand the reasoning behind AI-driven recommendations to maintain trust and make informed clinical judgments. Addressing these ethical considerations is essential for the responsible and effective implementation of AI in post-market surveillance.

Looking towards the future, the role of AI in PMS is poised to expand even further. We can anticipate the development of increasingly sophisticated AI architectures capable of analyzing data in real-time, providing immediate insights into emerging safety trends. Furthermore, AI’s ability to learn from historical data will likely lead to enhanced predictive capabilities, allowing for the identification of potential safety issues before they become widespread, enabling proactive interventions. The integration of AI with individual patient data may also pave the way for personalized risk assessments, tailoring surveillance efforts to the specific needs and risk factors of each patient. Ultimately, the most effective approach will likely involve a collaborative partnership between AI systems and human experts, where AI handles the complex task of data analysis, and healthcare professionals provide crucial clinical judgment and oversight. This synergy between artificial intelligence and human expertise will be instrumental in shaping the future of medical product safety.



